HITECH Act
What is the HITECH Act?
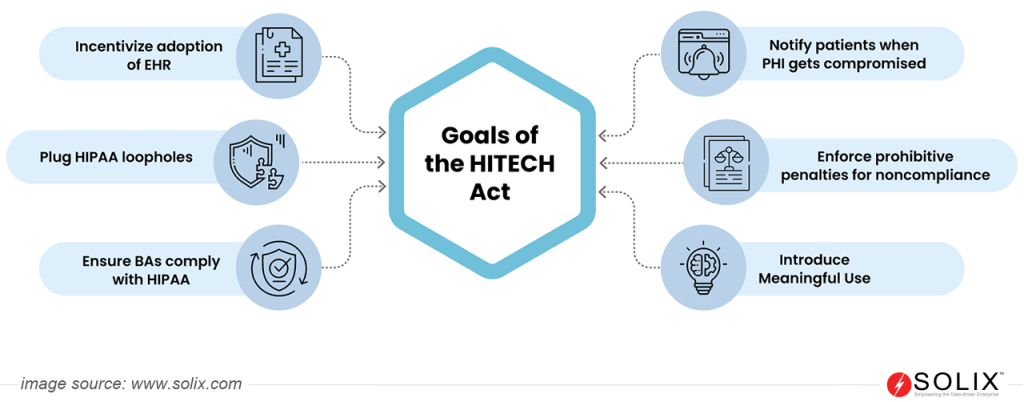
The Health Information Technology for Economic and Clinical Health (HITECH) Act is a part of the 2009 American Recovery and Reinvestment Act, aimed to boost electronic health records (EHR) adoption and strengthen healthcare data privacy and security. It offered financial incentives for meaningful use of EHRs and increased penalties for HIPAA violations. The Act also expanded HIPAA requirements to “business associates” handling patient data, boosting overall data protection.
Overview of the HITECH Act
- Law: Health Information Technology for Economic and Clinical Health Act
- Region: U.S.A
- Signed On: 17-02-2009
- Industry: Healthcare
Personal Data Under the HITECH Act
The HITECH Act was built upon the HIPAA privacy rule, so the personal data it protects is essentially the same: protected health information (PHI). In essence, any information a covered entity under HITECH creates, uses, or transmits that can be used to identify a patient and relates to their
- Medical history: Diagnoses, treatments, medications, allergies, and test results
- Payment information: Medical billing details, insurance information, co-pay amounts
- Demographic data: Name, address, phone number, date of birth, email addresses
- Genetic information: DNA data or other details about an individual’s genetic makeup
Key Components of the HITECH Act
- Financial Incentives: Encourages adopting Electronic Health Records (EHRs) through financial incentives, fostering improved healthcare quality and efficiency.
- Strengthened HIPAA Rules: Expands the reach of HIPAA to Business Associates, introduces Breach Notification Rule, and enhances enforcement mechanisms.
- Privacy and Security Standards: Defines specific data protection measures healthcare organizations must implement to safeguard patient information.
Data Protection Principle
Under the HITECH Act, healthcare organizations and their business associates must implement safeguards to protect PHI’s confidentiality, integrity, and availability. The HITECH Act upholds the core principles of HIPAA, focusing on
- Privacy: Patients can access, amend, and request restrictions on using their protected health information (PHI).
- Security: Healthcare organizations must implement reasonable and appropriate safeguards to protect PHI from unauthorized access, use, disclosure, alteration, or destruction.
- Breach Notification: Prompt notification is mandated for data breaches affecting 500 or more individuals to ensure timely mitigation and response.
Rights Under the HITECH Act
- Access to PHI: Patients can obtain a copy of their medical records in electronic format (if available) free of charge.
- Amendment of PHI: Request corrections to inaccurate or incomplete information in their medical records.
- Restrictions on Use and Disclosure: Limit the use and disclosure of their PHI for specific purposes.
- Accounting of Disclosures: Obtain a list of disclosures of their PHI made for purposes other than treatment, payment, or healthcare operations.
Who Needs to Comply with the HITECH Act?
The HITECH Act applies to two main categories of entities:
1. Covered Entities: These entities are already defined as “covered entities” under the HIPAA Privacy Rule. They include
- Healthcare providers: Hospitals, clinics, physicians, dentists, chiropractors, mental health professionals, nursing homes, etc.
- Health plans: Insurance companies, HMOs, and government health programs like Medicare and Medicaid.
- Healthcare clearinghouses: Entities that process healthcare information for multiple covered entities.
2. Business Associates: The HITECH Act significantly expanded the scope of HIPAA compliance by introducing the concept of “Business Associates.” Any entity that receives, transmits, or maintains Protected Health Information (PHI) on behalf of a covered entity must comply with the HITECH Act, even if they don’t directly treat patients or manage health plans. This encompasses various businesses, such as:
- Third-party administrators (TPAs) of health plans.
- Billing companies and medical coders.
- IT service providers and cloud computing vendors.
- Marketing and advertising agencies that handle healthcare data.
- Research institutions conducting studies using PHI.
It’s important to note that even subcontractors of business associates are also subject to compliance if they access or transmit PHI.
Noncompliance Fines
The HITECH Act established a tiered penalty system with varying levels of fines based on the culpability associated with the violation:
- Tier 1 (Reasonable Cause): $100 – $50,000 per violation, capped at $100,000 annually for identical violations.
- Tier 2 (Neglect): $1,000 – $50,000 per violation, capped at $1.5 million annually for identical violations.
- Tier 3 (Willful Neglect): $10,000 – $50,000 per violation, capped at $1.5 million annually for identical violations.
- Tier 4 (Corrected Action Needed): $50,000 per violation, capped at $1.5 million annually for identical violations.
Compliance Authority for the HITECH Act
The Office for Civil Rights (OCR) within the U.S. Department of Health and Human Services (HHS) is responsible for enforcing the act and investigating complaints of noncompliance.
In conclusion, understanding and adhering to the provisions of the HITECH Act are imperative for safeguarding electronic health information and maintaining compliance within the healthcare industry. Organizations can mitigate the risk of data breaches, uphold patient confidentiality, and avoid costly fines by prioritizing data protection principles, rights, and compliance measures outlined in the HITECH Act.
FAQs
Does HITECH require all healthcare providers to use electronic health records (EHRs)?
No, HITECH doesn’t mandate EHR adoption for all providers. However, it incentivized the switch to EHRs by offering financial rewards for “meaningful use.” These rewards have largely been phased out, but many providers have already adopted EHRs due to the benefits and potential penalties for not using them effectively.
What happens if a healthcare provider experiences a data breach under HITECH?
HITECH mandates that covered entities report certain data breaches to affected individuals and the Department of Health and Human Services (HHS), depending on the severity of the breach and the risk to patients.
Does HITECH apply to data collected in medical research studies?
HITECH generally doesn’t apply directly to research data, but research involving identifiable patient data might need to comply with HIPAA and its privacy rules. Additional institutional review board (IRB) approval processes might also be required for research studies.





